Blood Pressure Medication Recall 2021
Blood pressure medication is being recalled by the US. Blood pressure and fluid retention drugs recalled over cancer concerns.
09 Aug 2021 - 1318.

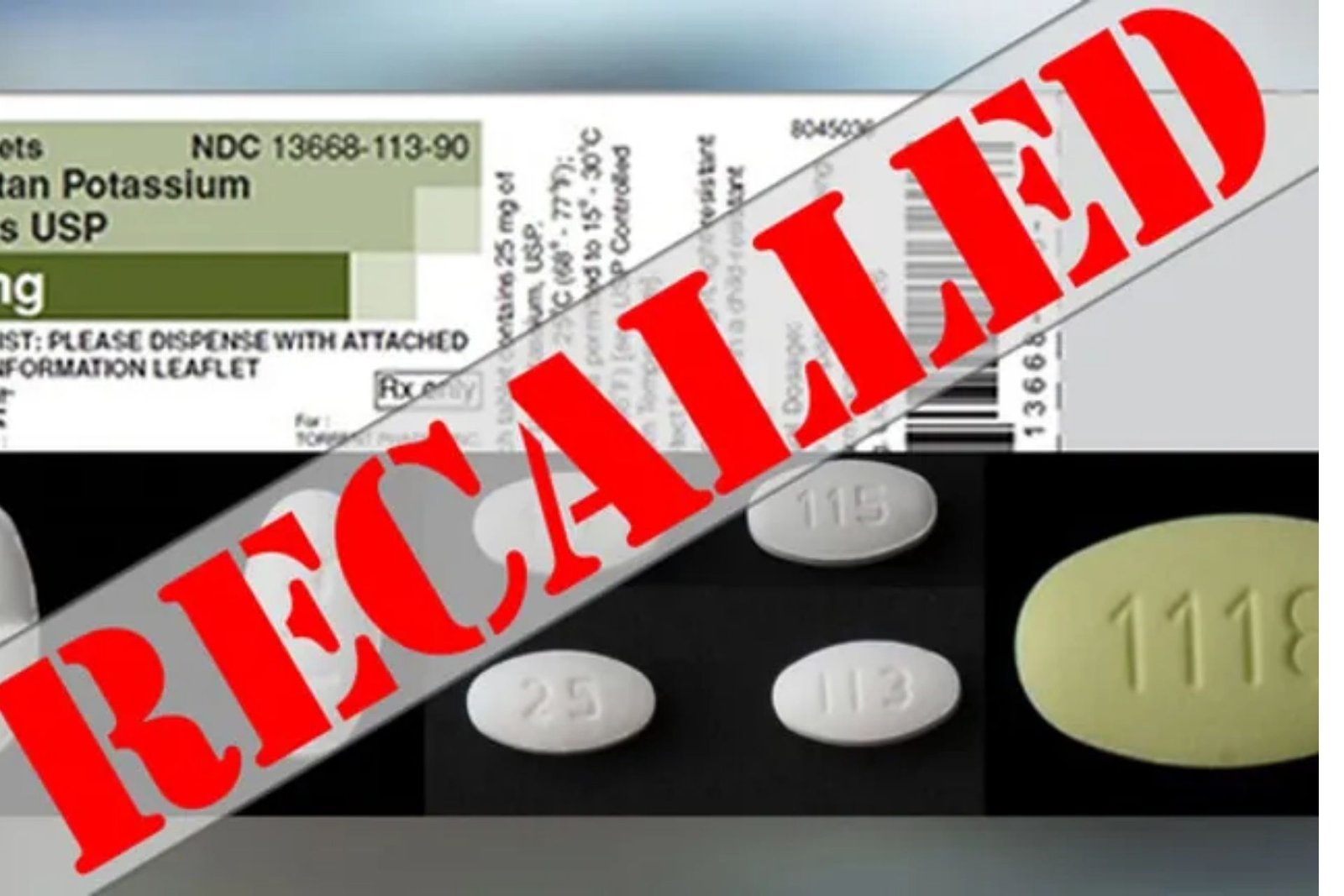
Blood pressure medication recall 2021. Irbesartan is a prescription-only medicine used to treat high blood pressure. Food and Drug Administration for possibly containing a cancer-causing impurityThe voluntary recall includes the firms Irbesartan tablets and Hydrochlorothiazide tablets at the consumer level according to the FDA. Blood pressure medication recalled over possibly containing cancer-causing impurity 0 shares.
They warned that suddenly stopping medication for high blood-pressure can be risky. Hypertension Drug Recalled Over Potential Death Risk. By Dawn Geske 032521 AT 1015 AM.
A recall is a voluntary action taken by a company at any time to remove a defective drug product from the market. Food and Drug Administration FDA for potentially containing a probable human carcinogen The voluntary recall includes the companys Irbesartan tablets and Hydrochlorothiazide tablets at the consumer level. Facebook Twitter LinkedIn Pinterest.
AVAPRO 75 mg TABLETS. The list below includes voluntary. AVALIDE 300-125 mg TABLETS.
AVALIDE 150-125 mg TABLETS. Find out which specific blood pressure medications are affected by the recall Search List of Recalled Angiotensin II Receptor Blockers ARBs including Valsartan Losartan and Irbesartan. Updated October 18 2021 756 AM.
Food and Drug Administration FDA. Batches of high blood pressure drug recalled due to contamination. Tuesday October 5 2021.
The FDA said Lupin Pharmaceuticals Inc. Blood Pressure Medication Recall 2021. Several companies are recalling Irbesartan Iosartan and valsartan due to the presence of an azido impurity at levels above what is considered safe.
5 hours agoThe US Food and Drug Administration has announced a recall of several batches of a blood pressure medication over concerns that it may contain high levels of a cancer-causing impurity. Two kinds of blood pressure medication recalled for possibly too much of a carcinogen By David J. Leave a Comment Lawsuits By Amy Gilmore.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. Is being recalled by the US. Patients prescribed angiotensin II receptor medications have filed Losartan recall lawsuits against drug manufacturers.
Losartan Recall Lawsuit 2021 Blood Pressure Drug Recalled. By Stephen Matthews Health Editor For Mailonline. Roston - Oct 15 2021 744pm CDT.
The company recalled all. A Lupin Pharmaceuticals Inc. Is recalling two types of blood pressure medication because the drugs may contain high levels of a substance that could cause cancer.
18 2021 -- Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential high. The four medications are. Food and Drug Administration FDA.
Cleveland 5 mins ago. 1157 EDT 9 August 2021. Tuesday October 19 2021 1004.
1040 17 Jun 2021. Blood Pressure Medication Recall 2021. A Lupin Pharmaceuticals Inc.
0710 EDT 9 August 2021 Updated. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer. The drugmaker received four reports of illness from Irbesartan and zero reports from the Irbesartan and Hydrochlorothiazide medication from the dates of Oct.
The UK medicine regulator today issued a. October 19 2021 704 AM 2 min read. Posted October 19 2021 234 pm EDT Has been updated.
Food and Drug Administration FDA for potentially containing a probable human carcinogen. A recall has been issued by Alembic Pharmaceuticals for one lot of. Hypertension Drug Recalled Over Potential Death Risk.
Brodies today asked customers to return four high blood pressure medications from Denk Pharma made in Germany. Doctors regularly prescribed antihypertensive drugs like Losartan Valsartan and Irbesartan to millions of patients in the. The FDA in a news release last week said that a.
The FDA has published a new recall advisory from Lupin Pharmaceuticals over. Blood pressure medication made by Lupin Pharmaceuticals Inc. Blood pressure medication is being recalled by the US.
Blood pressure medication recalled over risk of cancer-causing impurity Source link Blood pressure medication recalled over risk of cancer-causing impurity.

Blood Pressure Medication Recalled Because May Contain Stronger Dose Than Indicated Pennlive Com

Blood Pressure Medication Recall 2021 Hypertension Drug Recalled Over Cancer Risks

Blood Pressure Drug Recall Expands Again

High Blood Pressure Drug Recalled Because Labels Were Mixed Up Slashgear

Common Blood Pressure Drugs Recalled Over Fears Ingredient Increases Cancer Risk

Health Chiefs Recall Dozens Of Batches Of Common Blood Pressure Pills Daily Mail Online
Some Batches Of High Blood Pressure Medicine Were Recalled In Aruba Caribbean News Global

Blood Pressure Medication Recall 2021 Hypertension Drug Recalled Over Cancer Risks

Batches Of High Blood Pressure Drug Recalled Due To Contamination Derry Journal